Metabolic alkalosis
Published: 18 Jun 2025
ICD9: 276.3 ICD10: E87.3 ICD11: 5C74
Metabolic alkalosis is a condition where your body has too much bicarbonate (HCO3-) in relation to the amount of acid.
In simpler terms, it's a disturbance in your body's acid-base balance where your blood becomes too alkaline (high pH).
Here's a breakdown of the key elements:
![]() Alkalosis: Refers to a condition where the blood pH is higher than normal (above 7.45).
Alkalosis: Refers to a condition where the blood pH is higher than normal (above 7.45).
![]() Metabolic: Indicates that the primary cause is related to a metabolic problem, not a respiratory problem. "Metabolic" refers to the chemical processes that occur within the body.
Metabolic: Indicates that the primary cause is related to a metabolic problem, not a respiratory problem. "Metabolic" refers to the chemical processes that occur within the body.
![]() Bicarbonate (HCO3-): A base that helps regulate the blood's pH. Metabolic alkalosis is characterized by an *increase* in bicarbonate levels.
Bicarbonate (HCO3-): A base that helps regulate the blood's pH. Metabolic alkalosis is characterized by an *increase* in bicarbonate levels.
![]() Acid: The opposite of a base. An example is hydrochloric acid (HCl) in the stomach. Metabolic alkalosis can also result from a *loss* of acid.
Acid: The opposite of a base. An example is hydrochloric acid (HCl) in the stomach. Metabolic alkalosis can also result from a *loss* of acid.
![]() pH: A measure of how acidic or alkaline a solution is. The normal pH range for arterial blood is 7.35 to 7.45.
pH: A measure of how acidic or alkaline a solution is. The normal pH range for arterial blood is 7.35 to 7.45.
Causes of Metabolic Alkalosis:
Metabolic alkalosis can arise from a variety of factors, including:
![]() Loss of Acid:
Loss of Acid:![]()

![]() Vomiting: Prolonged and excessive vomiting removes stomach acid (hydrochloric acid, HCl), leading to a relative excess of bicarbonate.
Vomiting: Prolonged and excessive vomiting removes stomach acid (hydrochloric acid, HCl), leading to a relative excess of bicarbonate.![]()

![]() Gastric Drainage: Similar to vomiting, draining the stomach contents via a nasogastric (NG) tube removes acid.
Gastric Drainage: Similar to vomiting, draining the stomach contents via a nasogastric (NG) tube removes acid.![]()

![]() Chloride-Losing Diarrhea (Rare): In rare cases, specific types of diarrhea can lead to significant chloride loss, resulting in alkalosis.
Chloride-Losing Diarrhea (Rare): In rare cases, specific types of diarrhea can lead to significant chloride loss, resulting in alkalosis.
![]() Gain of Bicarbonate:
Gain of Bicarbonate:![]()

![]() Excessive Bicarbonate Administration: Giving too much bicarbonate, either intravenously or orally (e.g., antacids containing bicarbonate), can directly raise bicarbonate levels.
Excessive Bicarbonate Administration: Giving too much bicarbonate, either intravenously or orally (e.g., antacids containing bicarbonate), can directly raise bicarbonate levels.![]()

![]() Milk-Alkali Syndrome: Caused by consuming excessive amounts of calcium and absorbable alkali (like bicarbonate), typically to treat heartburn.
Milk-Alkali Syndrome: Caused by consuming excessive amounts of calcium and absorbable alkali (like bicarbonate), typically to treat heartburn.![]()

![]() Rapid Correction of Chronic Hypercapnia: In individuals with chronic respiratory problems who retain carbon dioxide (CO2), a sudden lowering of CO2 levels through mechanical ventilation can lead to a relative excess of bicarbonate.
Rapid Correction of Chronic Hypercapnia: In individuals with chronic respiratory problems who retain carbon dioxide (CO2), a sudden lowering of CO2 levels through mechanical ventilation can lead to a relative excess of bicarbonate.
![]() Kidney-Related Issues:
Kidney-Related Issues:![]()

![]() Diuretics: Certain diuretics (particularly loop and thiazide diuretics) can cause the kidneys to excrete more acid and retain more bicarbonate, contributing to alkalosis.
Diuretics: Certain diuretics (particularly loop and thiazide diuretics) can cause the kidneys to excrete more acid and retain more bicarbonate, contributing to alkalosis.![]()

![]() Contraction Alkalosis: Occurs when there's a significant loss of fluid volume (e.g., from diuretics or fluid shifts). The kidneys try to conserve sodium, leading to increased bicarbonate reabsorption.
Contraction Alkalosis: Occurs when there's a significant loss of fluid volume (e.g., from diuretics or fluid shifts). The kidneys try to conserve sodium, leading to increased bicarbonate reabsorption.![]()

![]() Hyperaldosteronism: Excess aldosterone (a hormone) causes the kidneys to retain sodium and excrete potassium and hydrogen ions, leading to alkalosis.
Hyperaldosteronism: Excess aldosterone (a hormone) causes the kidneys to retain sodium and excrete potassium and hydrogen ions, leading to alkalosis.
![]() Other:
Other:![]()

![]() Bartter Syndrome and Gitelman Syndrome: Rare genetic disorders that affect the kidneys' ability to reabsorb electrolytes, leading to alkalosis.
Bartter Syndrome and Gitelman Syndrome: Rare genetic disorders that affect the kidneys' ability to reabsorb electrolytes, leading to alkalosis.
Symptoms of Metabolic Alkalosis:
The symptoms of metabolic alkalosis can vary depending on the severity and underlying cause. Some people may have no symptoms at all. Common symptoms include:
![]() Neurological:
Neurological:![]()

![]() Confusion
Confusion![]()

![]() Lightheadedness
Lightheadedness![]()

![]() Muscle weakness
Muscle weakness![]()

![]() Muscle cramps or spasms
Muscle cramps or spasms![]()

![]() Tetany (muscle spasms, often in the hands and feet)
Tetany (muscle spasms, often in the hands and feet)![]()

![]() Seizures (in severe cases)
Seizures (in severe cases)
![]() Respiratory:
Respiratory:![]()

![]() Slow and shallow breathing: The body tries to compensate by retaining CO2 to lower the pH.
Slow and shallow breathing: The body tries to compensate by retaining CO2 to lower the pH.
![]() Cardiovascular:
Cardiovascular:![]()

![]() Arrhythmias (irregular heartbeats)
Arrhythmias (irregular heartbeats)
![]() Gastrointestinal:
Gastrointestinal:![]()

![]() Nausea and vomiting (especially if caused by vomiting)
Nausea and vomiting (especially if caused by vomiting)
Diagnosis:
Metabolic alkalosis is diagnosed through blood tests, specifically:
![]() Arterial Blood Gas (ABG): This is the primary test. It measures:
Arterial Blood Gas (ABG): This is the primary test. It measures:![]()

![]() pH: Elevated (above 7.45) in alkalosis.
pH: Elevated (above 7.45) in alkalosis.![]()

![]() PaCO2 (partial pressure of carbon dioxide): May be normal or elevated, depending on the body's attempt to compensate through hypoventilation.
PaCO2 (partial pressure of carbon dioxide): May be normal or elevated, depending on the body's attempt to compensate through hypoventilation.![]()

![]() HCO3- (bicarbonate): Elevated.
HCO3- (bicarbonate): Elevated.
![]() Serum Electrolytes: To assess potassium, chloride, and sodium levels. Hypokalemia (low potassium) and hypochloremia (low chloride) are often associated with metabolic alkalosis.
Serum Electrolytes: To assess potassium, chloride, and sodium levels. Hypokalemia (low potassium) and hypochloremia (low chloride) are often associated with metabolic alkalosis.
Treatment:
Treatment depends on the underlying cause and the severity of the alkalosis:
![]() Address the Underlying Cause: The most important step is to identify and treat the condition causing the alkalosis (e.g., stop vomiting, discontinue certain medications, treat hyperaldosteronism).
Address the Underlying Cause: The most important step is to identify and treat the condition causing the alkalosis (e.g., stop vomiting, discontinue certain medications, treat hyperaldosteronism).
![]() Restore Fluid and Electrolyte Balance:
Restore Fluid and Electrolyte Balance:![]()

![]() IV Fluids: Administering intravenous fluids, especially those containing sodium chloride, can help correct volume depletion and chloride deficiency.
IV Fluids: Administering intravenous fluids, especially those containing sodium chloride, can help correct volume depletion and chloride deficiency.![]()

![]() Potassium Replacement: If hypokalemia is present, potassium supplements (oral or IV) are given.
Potassium Replacement: If hypokalemia is present, potassium supplements (oral or IV) are given.
![]() Acid Administration (Rare): In very severe cases, administering hydrochloric acid (HCl) or ammonium chloride (NH4Cl) may be necessary to directly lower the pH. This is usually reserved for situations where other measures are ineffective and the alkalosis is life-threatening. This must be done very cautiously and with close monitoring.
Acid Administration (Rare): In very severe cases, administering hydrochloric acid (HCl) or ammonium chloride (NH4Cl) may be necessary to directly lower the pH. This is usually reserved for situations where other measures are ineffective and the alkalosis is life-threatening. This must be done very cautiously and with close monitoring.
![]() Acetazolamide: This is a carbonic anhydrase inhibitor that promotes bicarbonate excretion by the kidneys. It is used when fluid administration alone isn't enough or when there are contraindications to fluid administration (e.g., heart failure).
Acetazolamide: This is a carbonic anhydrase inhibitor that promotes bicarbonate excretion by the kidneys. It is used when fluid administration alone isn't enough or when there are contraindications to fluid administration (e.g., heart failure).
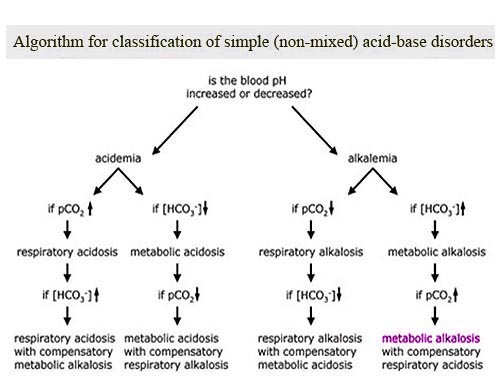
Compensation:
The body tries to compensate for metabolic alkalosis by:
![]() Hypoventilation: Slowing down breathing to retain CO2, which is an acid. This increases PaCO2.
Hypoventilation: Slowing down breathing to retain CO2, which is an acid. This increases PaCO2.
![]() Kidney Adjustments: The kidneys attempt to excrete more bicarbonate in the urine.
Kidney Adjustments: The kidneys attempt to excrete more bicarbonate in the urine.
However, the compensation is rarely complete, and the pH usually remains above normal.
In summary, metabolic alkalosis is a condition of abnormally high blood pH caused by an increase in bicarbonate or a loss of acid, and it requires a careful evaluation to determine the underlying cause and appropriate treatment.